All Features
Etienne Nichols
In the United States, the Food and Drug Administration (FDA) is the federal agency tasked with regulating the medical device market and ensuring the safety and effectiveness of all devices for patients.
The FDA classifies medical devices by risk into three categories: Class I, Class II, and Class…

Stephanie Ojeda
An analysis of U.S. Food and Drug Administration (FDA) warning letters by the Food and Drug Law Institute reveals a perhaps not-so-surprising link between training gaps and FDA violations.
It’s one of several factors motivating companies to switch to automated training management software. The…

Aman Pandey
In the dynamic landscape of the life sciences industry, ensuring compliance with good manufacturing practices (GMP) is imperative to guarantee the safety, efficacy, and quality of pharmaceutical products.
One critical aspect of GMP is the analytical product quality review (APQR), an essential…

Dawn Bailey
The Center for Organ Recovery & Education (CORE), a 2019 Baldrige Award recipient, is a nonprofit organ procurement organization (OPO) in Pittsburgh with a federally designated service area encompassing a population of 5.5 million in western Pennsylvania, West Virginia, and one county in New…

Patrick Gale
Medical equipment is a necessary yet substantial investment for any health system. Making strategic decisions about these assets can be daunting in the face of shifting patient demand, financial uncertainty, and fast-changing cybersecurity risks.
Because clinical assets account for an average of…
Alonso Diaz, Maria DiBari
The U.S. Food and Drug Administration (FDA) emphasizes the importance of being prepared for device recalls.
FDA product recalls are on the rise in the post-pandemic era. There has been a clear upward trend from 2021 through 2023, and medical devices ranked the highest of all product types. (See…

Maggie Overfelt
Michele Gelfand finds inspiration for new projects all around her: taking in the banter in a boardroom, speaking with taxi drivers when traveling, observing the interactions between physicians and nurses during an unexpected trip to the doctor. The idea for one of her most recent papers was sparked…

NIST
Stain-resistant clothing, fast-food wrappers, and extreme weather gear such as certain jackets and pants—these products get many of their desirable features from a class of manufactured chemicals called per- and polyfluoroalkyl substances (PFAS). But there’s a major downside: Researchers have found…

Stephanie Ojeda
As U.S. Supreme Court Justice Louis D. Brandeis famously wrote, “Sunlight is said to be the best of disinfectants.”
In the field of quality, internal audits are the equivalent of sunlight. Like spring cleaning, internal audits provide the opportunity to bring process issues into the open before…

Jamie Bihary
An internal audit can be an overwhelming prospect, especially if you’re new to a company or internal auditing in general.
The MedTech space is huge, and even the standards that are meant to help, like ISO 13485:2016, cover a lot of ground.
So, if you’re part of the audit team in your company, and…

Stephanie Ojeda
Formal complaint management is a requirement in regulated industries such as medical device and pharmaceuticals under U.S. Food and Drug Administration (FDA) and other international regulations.
The FDA mandates that medical device companies, for example, designate a formal unit for managing…

Michael King
In the ever-evolving life-sciences industry, market share is fiercely contested and companies must continuously optimize their operations to maintain their competitive edge.
Modern technologies and intelligence-driven solutions are revolutionizing how organizations work, empowering them to elevate…

Meg Sinclair
At Qualio, our mission is to help life science companies embed robust digitized quality to get their critical products to market at rapid speed and keep them there. And because the Qualio+ team combines over a century of collective quality and regulatory experience from within the life science…

Alonso Diaz, Maria DiBari
Inspections by the U.S. Food and Drug Administration (FDA) are on the rise after the nation has recovered from the Covid-19 pandemic. Domestic inspections showed a drop in 2020 due to state health guidelines around quarantine.
The rise has more than doubled within three years of post-pandemic…

ComplianceQuest
Today’s quality leaders in the life sciences industry have nearly impossible charters. Long-term trends and sudden black swan events in combination can hinder an organization’s ability to exert control over product quality. Globalization, labor shortages and strikes, outsourcing, just-in-time…

Kari Miller
Traditionally, quality management in the pharmaceutical industry has strayed away from artificial intelligence (AI) for fear of setting it loose with such sensitive information. They have been cautious of implementing an additional element of intelligence into their process. But will organizations…

Del Williams
To provide food processors with insight into the industry’s current challenges and opportunities, Cablevey Conveyors, a global specialty conveyor manufacturer, and Automated Handling Solutions, a service-focused subsidiary of Cablevey, have released results from an annual proprietary survey…

Stephanie Ojeda
There’s an old saying in regulated industries: If it isn’t documented, it didn’t happen.
In the past, maintaining fully compliant documentation meant handling a mountain of paper, which created extra work—and hidden risks—from a quality perspective.
Today, document management has become the…
Jón Bergsteinsson
Clinical investigations play an important role in your journey of bringing a medical device to market. While the relevant standards are often perceived as difficult and complex, having a good grasp of them makes the process less confusing.
Understanding ISO 14155:2020 is essential. It’s a guide to…

Stephanie Ojeda
In December 2023, the U.S. Food and Drug Administration (FDA) expects to issue its long-awaited overhaul of its Quality System Regulation (QSR). The biggest change is that the new Quality Management System Regulation (QMSR) will harmonize with ISO 13485 for medical device quality management. With…
Steve Thompson
If you’ve ever enjoyed the experience of an audit or inspection, then you know it’s about as much fun as having your wisdom teeth extracted. As painful as audits and inspections may be, they are necessary to bring needed medical products to market and monitor them to protect consumers and patients…

Kelley Jacobsen
In the wake of the Covid-19 pandemic, medical device supply chains are one of the top priorities for health system leaders. Amid supply chain disruptions during the pandemic, hospitals scrambled to find enough devices to keep up with unprecedented demand. The global crisis revealed gaps in standard…
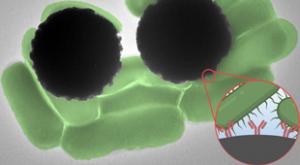
Jennifer Chu
Getting blood test results can take anywhere from a day to a week, depending on what a test is targeting. The same goes for tests of water pollution and food contamination. And in most cases, the wait time has to do with time-consuming steps in sample processing and analysis.
Now, MIT engineers…

Matthew M. Lowe
Let’s start with a definition of Industry 4.0, keeping in mind that we’re rapidly approaching Industry 5.0. Industry 4.0 is an era marked by enhanced digitization and the increased connectivity of smart technologies. Where Industry 5.0 is more values-driven, it will require the technology of…
Etienne Nichols
On Feb. 23, 2022, the U.S. Food and Drug Administration (FDA) released its proposed rule for the new Quality Management System Regulation (QMSR). The proposed QMSR will be the result of aligning the current good manufacturing practice (cGMP) requirements of the FDA’s Quality System Regulation (QSR…