Selecting
Document
Control Software by Adrian Burr There is a growing realization in both public and private sectors
that effective, continuous improvement requires efficient
and effective information systems. One of the first comments from a company developing or using a quality system concerns the volume of paperwork involved. In fact, organizations must deal with two paper trails when initiating or maintaining an ISO 9000 or QS-9000 quality system. The first trail accumulates from the standard's requirement that the system be documented and quality records generated and maintained. The second occurs when the company or the standards change, when better practices are used or any other changes are made: All the existing documentation and paperwork must be changed, too. Document control software originally was developed to automate these processes. However, over the last five years, software has explored several other application areas. For instance, it's no longer necessary to document parallel definitions of an organization and its activities. This can now be documented as a company process map, which provides the activity interface for every individual or department and automatically generates the quality records as work is completed, thus embedding quality information systems into work activities. Such software has enabled many companies to comply with the original concepts behind the standards initiated by Walter Shewhart, W. Edwards Deming, Philip Crosby and Joseph Juran. These concepts have been further developed in the Baldrige Award, the Software Engineering Institutes Capability Maturity Model, the European Foundation for Quality Management model and in the other models developed by both large and small consultancy organizations. Software has also become the means for capturing and measuring best practices, detecting nonconformances, managing improvement, modeling processes, gathering capability information and providing training. Early on, ISO 9000 suffered from the problem of companies becoming certified in order to convince a customer that they are quality suppliers. Computers were not as powerful or accessible as they are now, which made sharing information difficult and issuing and releasing documents time-consuming and expensive. It is little wonder that quality standards were criticized for putting bureaucracies into place rather than providing a model for effective management. However, there is a growing realization in both public and private sectors that effective, continuous improvement requires knowledge of the processes, which in turn requires efficient and effective information systems. The U.S. General Services Administration was the first U.S. government service organization to achieve ISO 9000 certification. Stewart J. Levy, co-chairman of the implementation team, in a meeting in December 1997 said that the GSA is interested in expanding the ISO 9000 process throughout its organization nationwide. It is also considering giving preference to ISO 9000-registered suppliers and service providers. This was part of GSA's decision to become a certified organization itself. "If we decided to give a preference to ISO 9000-registered companies, we felt we needed to be registered ourselves," says Levy. The GSA used software to increase the effectiveness of its ISO 9000 quality system, one of the obvious benefits along with identifying and simplifying activities and functions. Future quality standards issues are expected to embrace process and improvement even more firmly than current versions do. The principles of process modeling -- where a process is any activity with inputs and outputs, including management, sales, financial control, administrative actions, service operations and manufacturing -- are likely to be built into future versions of ISO 9000. QS-9000 already provides a model that includes many process control principles and could serve as the basis of the new standard. Whether this becomes the case or not, a company intent on making effective use of ISO 9000 certification will want to use the best tools available to maximize return on investment. Major organizations worldwide are starting to use these process improvement principles. One quality manager has defined best practice as "identifying the essential processes for the business and putting into place an information system that will provide management with the tools to optimize performance for delivery of product or service to the customer, for quality of life of the employees and for maximum return for the stockholders." Matching software with company cultures A mature, optimized company evolves through six stages:  No quality system in place, no procedures or work instructions No quality system in place, no procedures or work instructions
 Quality systems operational but not conforming to any standard Quality systems operational but not conforming to any standard
 ISO 9000-conforming manual system ISO 9000-conforming manual system
 ISO 9000-conforming automated system ISO 9000-conforming automated system
 Quality improvement in place Quality improvement in place
 Capability management in place Capability management in place
Any company will start out somewhere within this process. Software is used initially to help implement quality management, automate documentation control and delivery, provide information about performance and conformance, and manage improvement. These packages are known generically as quality management support systems. What software a company will purchase depends on its own quality development. A company just beginning to think in terms of quality will require tools to help identify where ISO 9000 applies to the company. Appropriate training tools, project implementation tools and assessment systems also are requirements. If an organization already has a quality management support system in place, then it will require software to assist in running and organizing that system, including version control and document distribution. Tools to manage work flow and identify and manage processes may replace the need for document management systems. If the company is trying to improve and optimize its capability, then process identification and improvement tools are essential. The second consideration for software selection is the organization's ability to make and absorb changes -- i.e., its change maturity. This is categorized as six levels:  Ad hoc. Change management is nonexistent or sporadic. Ad hoc. Change management is nonexistent or sporadic.
 Stable. Company has stable management but no formal change management. Stable. Company has stable management but no formal change management.
 Changeable. Company is able to receive and use new methods. Changeable. Company is able to receive and use new methods.
 Improving. Process improvement is in place and already used. Improving. Process improvement is in place and already used.
 Capable. Change management is used to improve the company. Capable. Change management is used to improve the company.
 Optimized. Company makes changes to optimize capability. Optimized. Company makes changes to optimize capability.
By combining quality systems maturity and change maturity, organizations can select software types as shown in Table 1. Identifying software types allows organizations to select software best suited to their developmental level. 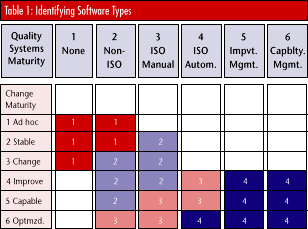
Different industries emphasize different requirements. The industry categories are:  General manufacturing for retail and trade other than the following categories General manufacturing for retail and trade other than the following categories
 Automotive (QS-9000) manufacturers and their supplier companies Automotive (QS-9000) manufacturers and their supplier companies
 Service, including financial, property, transport, vacation and retirement homes Service, including financial, property, transport, vacation and retirement homes
 Hospitals, doctors, pharmacies and pharmaceutical companies supplying the medical industry Hospitals, doctors, pharmacies and pharmaceutical companies supplying the medical industry
 Food and beverage industry, from producers to supermarkets Food and beverage industry, from producers to supermarkets
 Software industry (although as they mature, package software developers and systems maintenance companies gradually are being recognized as a general service industry) Software industry (although as they mature, package software developers and systems maintenance companies gradually are being recognized as a general service industry)
 Research and development companies Research and development companies
 Military establishments Military establishments
 High-risk industries such as aerospace and nuclear power plants High-risk industries such as aerospace and nuclear power plants
Different levels of software are available for each of these industry types. A software's flexibility can influence system selection as organizations seek to match company culture and procedures with a program's functions. Most companies can use existing packages and provide minimum manual operations to match the company culture to the system. For companies with very different internal cultures and information flows, the software may have to be written specifically for the organization. Available software will run on most platforms commonly used for management information systems today. There are specific systems for Windows and UNIX as well as for Lotus Notes. These systems provide appropriate functions, including intranet capability rather than standard shared networks. In fact, software changes over the last half of 1997 have all addressed the need to provide document viewers and generators for HTML formats for information sharing, even across international boundaries. Software packages Product selection can be narrowed by using Tables 1 and 2. A level 1/1 company -- i.e., no current systems, no change management -- requires a startup system for development, training and product assessment. The development system should include ISO 9000 text, conformance assessment, quality system content guidelines and assistance with quality manuals and procedures. 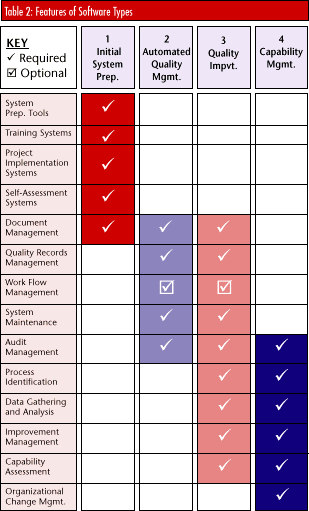
Once past that stage, organizations select products that either are general in nature or provide specific tools for the industry sector in question. Finally, the company should decide whether it is willing to fit its organization to a system or whether the system should be designed to match current company operations and culture. For a more mature company, the selection might involve level 3 (a manual quality system in place) or level 4 (change management in place and in use). Software for such a company is categorized as either automated quality management or quality improvement. Most companies, intent on improving current capability, will select quality improvement. Organizations desiring to reduce document management overheads will select programs within the automated quality management section. A level 1/1 company might consider the following packaged software solutions:  Preparation systems: DocISO, ISOPro, ProcessExpert, mPower, Architect, SHL Transform Preparation systems: DocISO, ISOPro, ProcessExpert, mPower, Architect, SHL Transform
 Training tools: CBT, How to Implement ISO 9000, Lasermedia, Quality in Practice, SHL Transform, ISOInfo, TQM CD, TQMPro Training tools: CBT, How to Implement ISO 9000, Lasermedia, Quality in Practice, SHL Transform, ISOInfo, TQM CD, TQMPro
 Project management: standard PM tools, TOPQ Project management: standard PM tools, TOPQ
 Assessment systems: The Quality Engine, RapidScore, ISO Audit, TOPQ Assessment systems: The Quality Engine, RapidScore, ISO Audit, TOPQ
Whereas a company at level 3/4 might consider these products:  Document management: PowerWay DocWriter, TDOC, Frame Document management: PowerWay DocWriter, TDOC, Frame
 Process identification systems: QMap, Witness, ProcessExpert, SHL Transform, Architect, FlowMAP, 4TQFlowPlus, TeamFlow, oCTAVe, Optima, ProcessWise, Simmit! Process identification systems: QMap, Witness, ProcessExpert, SHL Transform, Architect, FlowMAP, 4TQFlowPlus, TeamFlow, oCTAVe, Optima, ProcessWise, Simmit!
 Work flow management: Quality Workbench, Q-Pak, IQS, Q-Pulse, System 9000, Qualibaz, Paradigm Quality, Aimware, EQS, TriangleQA, Q-SYS QA Work flow management: Quality Workbench, Q-Pak, IQS, Q-Pulse, System 9000, Qualibaz, Paradigm Quality, Aimware, EQS, TriangleQA, Q-SYS QA
 Data gathering and analysis systems: QIS9000, HMS, RQM as well as standard SPC packages Data gathering and analysis systems: QIS9000, HMS, RQM as well as standard SPC packages
 Improvement management: Action, Quest, KnowledgeSeeker, Benchmarker Improvement management: Action, Quest, KnowledgeSeeker, Benchmarker
 Assessment and capability: The Baldrige Award, The Quality Engine, TOPQ, QIS9000, SPC Benchmarker Assessment and capability: The Baldrige Award, The Quality Engine, TOPQ, QIS9000, SPC Benchmarker
The product examples above by no means constitute a comprehensive listing. More than 100 products are on the market; product specifications are available from the tMSc review report Cutting Through the Paperwork or by searching the market and identifying products that have the characteristics described. The examples below list features usually included in a typical package within a specific category (see Table 2). Each package differs in its approach to the problem.  Initial system preparation -- Systems in this category provide documentation guidelines, completeness analysis, project status, version control and issue management. They also provide process mapping systems, training, project management and self-assessment systems. Initial system preparation -- Systems in this category provide documentation guidelines, completeness analysis, project status, version control and issue management. They also provide process mapping systems, training, project management and self-assessment systems.
 Automated quality management -- Automated QMS systems provide tools for quality managers and include document management, audit scheduling and internal assessments. Alternatively, a work flow system will automate an organization's actual activities, providing on-line process functions, guidelines, documentation and quality information. Automated quality management -- Automated QMS systems provide tools for quality managers and include document management, audit scheduling and internal assessments. Alternatively, a work flow system will automate an organization's actual activities, providing on-line process functions, guidelines, documentation and quality information.
 Continuous quality improvement -- Most standard quality improvement tools fall into this category; corporate quality information databases are fed from quality function deployment, statistical process control and failure mode effects analysis systems. Logging systems manage improvement activities at all levels, while quality costing identifies improvement benefits. Continuous quality improvement -- Most standard quality improvement tools fall into this category; corporate quality information databases are fed from quality function deployment, statistical process control and failure mode effects analysis systems. Logging systems manage improvement activities at all levels, while quality costing identifies improvement benefits.
 Capability management -- A mature and stable company committed to quality management methods will employ systems in this category. Capability management programs use the periodically compiled data to analyze processes' effectiveness and efficiency. Capability management -- A mature and stable company committed to quality management methods will employ systems in this category. Capability management programs use the periodically compiled data to analyze processes' effectiveness and efficiency.
There are now software packages available that combine health, safety and en-vironmental analysis with quality management. Many systems also include programs to manage available skills within an organization. Quality management vs. information management Many companies run two information systems within their organizations -- one for information management and one for quality management. Management information systems often are automated using standard document control and work management software. However, most of these systems only provide mechanisms for doing the work, not for controlling improvement, change management, or issue and release control. Increasingly, companies are offering software to fill this gap. The tMSc report reviews more than 100 available packages that provide such control mechanisms. Most use standard database systems. Together with the work management systems for activities such as calibration and testing, these new systems form the bulk of the software available to assist companies in developing effective information systems based upon principles of continuous improvement. These packages link with other standard systems to complete the work and provide a common interface and access to information as well as a common method of producing quality records for process improvement. The linked systems then provide an information system that conforms to quality standards. What to ask a software supplier When purchasing software, organizations should ask the same questions as for any new product. Considerations include:  Internal questions -- What functions are required? What is the cost justification for the purchase? What system compatibility is required to comply with internal information technology strategy? Once the software is installed, will it be possible to change suppliers or systems in the future? Internal questions -- What functions are required? What is the cost justification for the purchase? What system compatibility is required to comply with internal information technology strategy? Once the software is installed, will it be possible to change suppliers or systems in the future?
 Questions for the supplier -- Is the system year 2000 compliant, tolerant or fully functional? (Compliant systems will continue to function with future upgrades; tolerant systems will operate but aren't year 2000 compliant). How are new functions added? Is the system compatible with other packages? Is it possible to use and/or import existing documents? Questions for the supplier -- Is the system year 2000 compliant, tolerant or fully functional? (Compliant systems will continue to function with future upgrades; tolerant systems will operate but aren't year 2000 compliant). How are new functions added? Is the system compatible with other packages? Is it possible to use and/or import existing documents?
 Quality audit questions -- How often, on average, will the system software fail? How are software faults handled? What is the upgrade policy? Are all upgrades backward compatible? Can the supplier deliver improved code? (Best practice for these packages currently is around 25 defects per million lines of code, or one defect per million in the aerospace industry). Quality audit questions -- How often, on average, will the system software fail? How are software faults handled? What is the upgrade policy? Are all upgrades backward compatible? Can the supplier deliver improved code? (Best practice for these packages currently is around 25 defects per million lines of code, or one defect per million in the aerospace industry).
Once an organization decides on a purchase, suppliers should provide a list of required information for configuration purposes. This list usually includes the people who will use the system, the current company machine list, the company structure, who is responsible for issue and release of which parts of the quality documentation. Also included is the approved list of document checkers, internal auditors and suppliers as well as current projects and associated information such as leader, approver and sign-off authority. Most companies will be able to provide an itemized list and the format required for the simplest transformation from existing computer-based records and the new system. With a clear understanding of its own change maturity as well as current software packages, a company can expect to make effective and, ultimately, profitable document control software decisions. About the author Adrian Burr started tMSc in 1991 to provide statistical and quality methods consulting to industry and commerce. Burr is the author of Cutting Through the Paperwork, a review of quality management support, and co-author of Statistical Methods for Software Quality: Using Metrics for Process Improvement. For more information, contact Adrian Burr at tMSc, 13 Helions Road, Steeple Bumpstead, Haverhill, Suffolk, CB9 7DU, United Kingdom; telephone +44 1440 730211, fax +44 1440 730711, e-mail aburr@globalnet.co.uk. Or visit the company's Web site at www.globalnet.co.uk/~tMSc. |


