by John R. Grum
In their ongoing quest to build
a better mousetrap, software developers have combined calibration
management with the latest business process developments
to create an improved system for controlling calibration
and maintaining measurement instrumentation. However, this
breakthrough technology is driven by stringent requirements
that companies ignore at their peril. Quality standards
such as ISO 9001, ISO/IEC 17025 and ISO/TS 16949; regulatory
requirements such as the FDA’s code of federal regulations’
requirements for current good manufacturing practices; and
everyday business goals such as profitability all have a
bearing on the latest trends in calibration management systems.
Similarly, compliance to ISO/IEC 17025 underlies many
manufacturing requirements. This standard, mandated by ISO/TS
16949 for calibration service providers, is gospel for original
equipment manufacturers. It includes calibration basics
along with environmental conditions, measurement uncertainty,
proper certificate preparation, calibration technician training
and technical competence, and standard operating procedures
such as change control.
For FDA-regulated companies, compliance to the FDA’s
cGMP drives overall manufacturing requirements, just as
compliance with 21 CFR Part 11 drives electronic records
requirements. These include using validated computerized
systems; retaining secure electronic records; system and
data security; system access control; using secure electronic
signatures; and user-independent, computer-generated, time-stamped
audit trails.
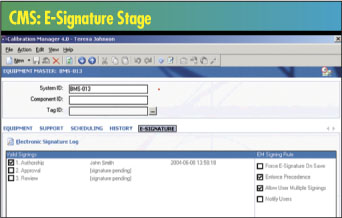
Historically, a CMS provided basic tracking, scheduling
and reporting functions. Modern packages offer flexibility
and customization to ensure they’ll fit into existing
calibration processes. New capabilities include tracking
full measurement, calibration and maintenance data; multiple-event
scheduling; multiple security levels; and attaching standard
operating procedures, certificates, charts and forms to
asset records.
New CMS features integrate the latest trends in calibration
management with those of business processes, including:
 Advancing software technologies
Advancing software technologies
 Assessing new architecture technologies
Assessing new architecture technologies
 Outsourcing calibrations
Outsourcing calibrations
 Collecting field data
Collecting field data
 Measuring performance
Measuring performance
 Centralizing calibration across the enterprise
Centralizing calibration across the enterprise
 Standardizing calibration between sites
Standardizing calibration between sites
As regulations become more defined and internal processes
are re-evaluated, a modern CMS can increase productivity,
manage compliance and improve your organization’s
return on its investment. However, upgrades in database,
report writing and operating system software must be accommodated
by the CMS.
The first step in migrating to a new system is evaluating
needs through a user-requirement specification. This is
a simple statement of what’s required of the system.
By defining the requirements necessary for the calibration
management process, an organization can narrow the search
for the CMS best suited to its needs. Guidelines for the
URS process include:
 Emphasizing the required function rather than the method
for implementing it
Emphasizing the required function rather than the method
for implementing it
 Writing a URS for each function the software will perform
Writing a URS for each function the software will perform
 Ensuring the URS will distinguish between regulatory requirements
and desirable features
Ensuring the URS will distinguish between regulatory requirements
and desirable features
Once the URS is completed, it can be evaluated against different
CMS packages and an educated decision made about which is
most suitable. Although the process takes time, it helps
to ensure a close match between the user’s requirements
and the CMS. Other considerations include flexibility, ease
of use, reliability and, most important, the technology
upon which the system is based.
The technology a CMS vendor uses to develop a software
package is crucial. Verifying that the CMS is designed for
Microsoft SQL Server or Oracle isn’t enough; organizations
must look at the type of architecture used. Growing companies
should evaluate whether the software package is scalable.
A CMS requires a significant investment of time and money,
and it can’t be changed every year to optimize productivity.
Therefore, manufacturing companies must evaluate the vendor’s
development practices to confirm they’re developing
not only for today but also for tomorrow.
CMS vendors are quick to take advantage of new technology
to produce state-of-the-art systems. Such software packages
provide not only best-in-class feature sets but also the
ability to share data in seconds with hundreds of users
from multiple locations.
The technology best equipped to satisfy these requirements
is an n-tiered Web model designed to distribute the necessary
user interaction, computation and storage tasks between
the layers of the architecture. Although some latitude exists
in the exact number and structure of layers, a CMS is typically
broken up into a client tier, a middle tier and a data store--which
is further segmented into a data abstraction layer and a
database server.
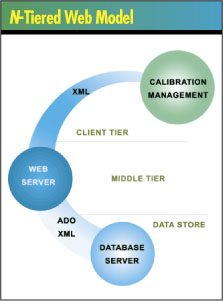  Client tier. This layer is responsible for interactions
with the user. In the past, all application users saw the
same interface. Today’s users access their data from
a variety of devices with various screen sizes and input
methods. The client tier must accommodate the user regardless
of the device bridging the user to the system.
Client tier. This layer is responsible for interactions
with the user. In the past, all application users saw the
same interface. Today’s users access their data from
a variety of devices with various screen sizes and input
methods. The client tier must accommodate the user regardless
of the device bridging the user to the system.
 Middle tier. Also known as the business logic
layer, the middle tier is where data are interpreted and
business rules applied. Certain types of security and access
checks are also performed at this level. The middle tier
is considered the brain of the n-tiered system; a Web server
offers the best performance.
Middle tier. Also known as the business logic
layer, the middle tier is where data are interpreted and
business rules applied. Certain types of security and access
checks are also performed at this level. The middle tier
is considered the brain of the n-tiered system; a Web server
offers the best performance.
 Data store. This tier includes the task of data
storage and retrieval. Once the choice of a database server
is made, a data abstraction layer is created to provide
the interface between the middle tier and the database itself.
Some popular databases include Microsoft SQL Server and
Oracle. For its reliability, MSDE is now frequently used
instead of Microsoft Access.
Data store. This tier includes the task of data
storage and retrieval. Once the choice of a database server
is made, a data abstraction layer is created to provide
the interface between the middle tier and the database itself.
Some popular databases include Microsoft SQL Server and
Oracle. For its reliability, MSDE is now frequently used
instead of Microsoft Access.
By using a Web-based, n-tiered architecture, CMS software
greatly reduces network traffic and the burden on client
resources while maintaining security and data integrity.
Users aren’t directly bound to the data store, as
they are with a traditional client-server application. Instead,
the middle tier uses connection pooling to enhance both
network and software resources. The result is improved performance.
In traditional Microsoft Access database applications,
the system slows down as more users log onto the software.
Data corruption is typical. With this modern architecture,
a CMS can handle hundreds of users without affecting performance.
Moreover, as an organization grows, so must its CMS. Application
scalability is very important; among other things, it means
tremendous cost savings when multiple sites are rolled into
one system.
One of the biggest trends in manufacturing is outsourcing
calibration chores. Limitations in time and human resources
generally motivate companies to consider this option. Because
a company might outsource all or only a few calibration
services, outsourcing itself falls into several categories.
The first consists of using a particular company to calibrate
specific equipment, which is gathered and routinely sent
off-site. The second involves having a company--such as
the manufacturer--service and calibrate the equipment. The
third is contracting a calibration service company to calibrate
the entire inventory on-site. Most companies use a combination
of these categories.
Regardless of who provides the calibration services, the
manufacturing company is responsible for managing and recording
calibrations. A state-of-the-art CMS provides the tools
to do this. It can track scanned certificates of completed
calibrations, equipment status and location, and reports
of money spent on outside calibrations. Metrics on service
provider performance are also important factors.
Collecting and managing both manufacturing and laboratory
data is an integral part of today’s calibration process.
A CMS must provide the capabilities to easily record such
data.
One of the best ways to collect field data is on a notebook
PC. Calibration technicians can transfer subsets of instrument
records from the main CMS into the notebook-based CMS module.
Calibration activities and events are then recorded in the
field on the notebook, and the data are transferred to the
main CMS. This process eliminates redundant data entry and
paper, greatly reducing the risk of error. A notebook PC
is ideal because it’s an easy-to-use format, powerful
enough to include standard operating procedures as well
as captured measurement data.
As the cost of notebook computers decreases and the advantages
of eliminating pen and paper are realized, a technologically
driven CMS that can provide security features and user options
along with a data-collection utility becomes increasingly
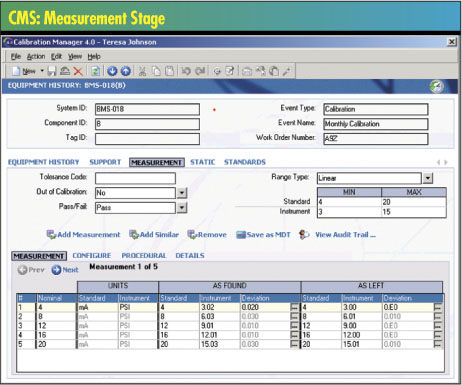
attractive. For paperless calibrations in regulated industries,
robust electronic signatures are also required at the point
of data collection.
Metrics used in calibration management have taken on new
importance as companies strive for global competitiveness.
Along with current software technology, key performance
indicators used in calibration management are proving helpful
in optimizing a range of calibration operations. The concept
of key performance indicators originated with equipment
and facilities maintenance, although the term is a familiar
one to computerized maintenance management system users
as well.
An enterprisewide CMS provides executive-level management
with a window on calibration operations, which in turn supports
decision making and productivity benchmarking. It’s
therefore not surprising that calibration budgets continue
to grow as compliance requirements become more stringent
and profitability goals more imperative. Companies are beginning
to see the benefits in handling KPIs in the same way they
handle their instrument calibrations. Business decisions
regarding equipment calibration and repair can be determined
from reliable data.
Controlling KPIs also can improve business performance
by optimizing maintenance intervals, reducing repair costs,
enhancing reliability and increasing productivity. A recent
survey from www.eCalibration.com
determined the top performance indicators in calibration
management. They are:
 Productivity measurement
Productivity measurement
 Project and capital justification
Project and capital justification
 Financial performance analysis
Financial performance analysis
 Customer satisfaction
Customer satisfaction
 Quality and regulatory issues
Quality and regulatory issues
Enterprisewide systems make the best use of new CMS technology.
For example, an enterprisewide CMS enables an organization
to host the application at a central location while allowing
worldwide access to separate departments and facilities.
In a typical installation, the database application is hosted
on centralized IT servers, configured and controlled by
a corporate quality or metrology group, and used by working
groups throughout the global enterprise. The software enables
each working group to maintain its own dataset configured
to its specific needs--including its own field labels, languages
or time zones--while centralizing the overall implementation
configuration.
An enterprisewide CMS can reduce calibration procedure
writing and management, standardize calibration measurement
data collection and retention, and allow for instant communication
of management and productivity metrics. This system also
functions as a platform for collaborative problem solving,
which is especially important in cases where employee input
is dispersed throughout the enterprise.
Companies that must follow standards or regulations will
quickly realize the advantages of an enterprisewide CMS.
All FDA-compliant companies must validate the software they
use when making products for public consumption. Validation
is an expensive and time-consuming task, but with an enterprisewide
implementation, it would be required only at the central
host site. If a CMS had been installed at each individual
site, then a validation would be necessary for each implementation.
An enterprisewide CMS can reduce validation costs by up
to 90 percent.
It can also add to the bottom line. Through cost reductions
in application licensing costs, corporate IT resources,
internal auditing and training, the enterprisewide CMS provides
the lowest total cost for corporate calibration compliance.
Although the enterprisewide approach standardizes calibration
processes throughout an organization, it’s also possible
to implement a CMS using a site-by-site method.
Not all companies can initially deploy a centrally hosted,
enterprisewide application. Divisions might want to remain
separate from others, an organization might be too diverse
or the infrastructure might not be capable of supporting
such a system. However, companies could require a single
CMS solution for all departments. In such cases, standardization
is the answer.
Individual departments within an organization can purchase
the same CMS, with each site hosting its own copy. This
can be beneficial in several ways. Each facility in a corporation
can purchase on its own schedule and within its own budget
constraints. Also, policies, procedures and processes can
be completely customized for each department. Finally, each
site can benefit from the knowledge and experience of other
sites within the corporation.
For companies whose ultimate goal is an enterprisewide
solution, the site-by-site approach with one CMS is a good
start. Given a well-researched standard technology, all
sites can easily be merged later into one comprehensive
package.
Historically, CMS vendors offered troubleshooting services,
infamously known as “technical support.” Today,
many CMS vendors offer turnkey service that includes project
assessment, system architecture design, systems implementation,
validation assistance, data import, report writing, commissioning
and training. Companies reduce costs and minimize implementation
risks by hiring vendor personnel to install and start up
systems quickly and efficiently.
Quality standards, regulatory requirements and business
goals drive the needs of today’s calibration management
systems. Modern CMS packages offer flexibility, customization
and a robust feature set to exceed those needs.
John R. Grum is the marketing manager at Blue Mountain
Quality Resources Inc. Blue Mountain is the validated leader
in calibration management software. With 15 years of experience
in FDA-regulated and ISO-compliant markets, Blue Mountain
offers a complete solution from site assessment and installation
to training and validation. For more information, go to
www.coolblue.com.
|