Design controls are a set of quality practices and procedures used to ensure that a finished device meets its user needs, intended use, and specified requirements.
|
ADVERTISEMENT |
The requirement for medical device companies to use design controls is established in 21 CFR Part 820, as well as ISO 13485:2016.
The classic design control “waterfall” diagram looks like this:
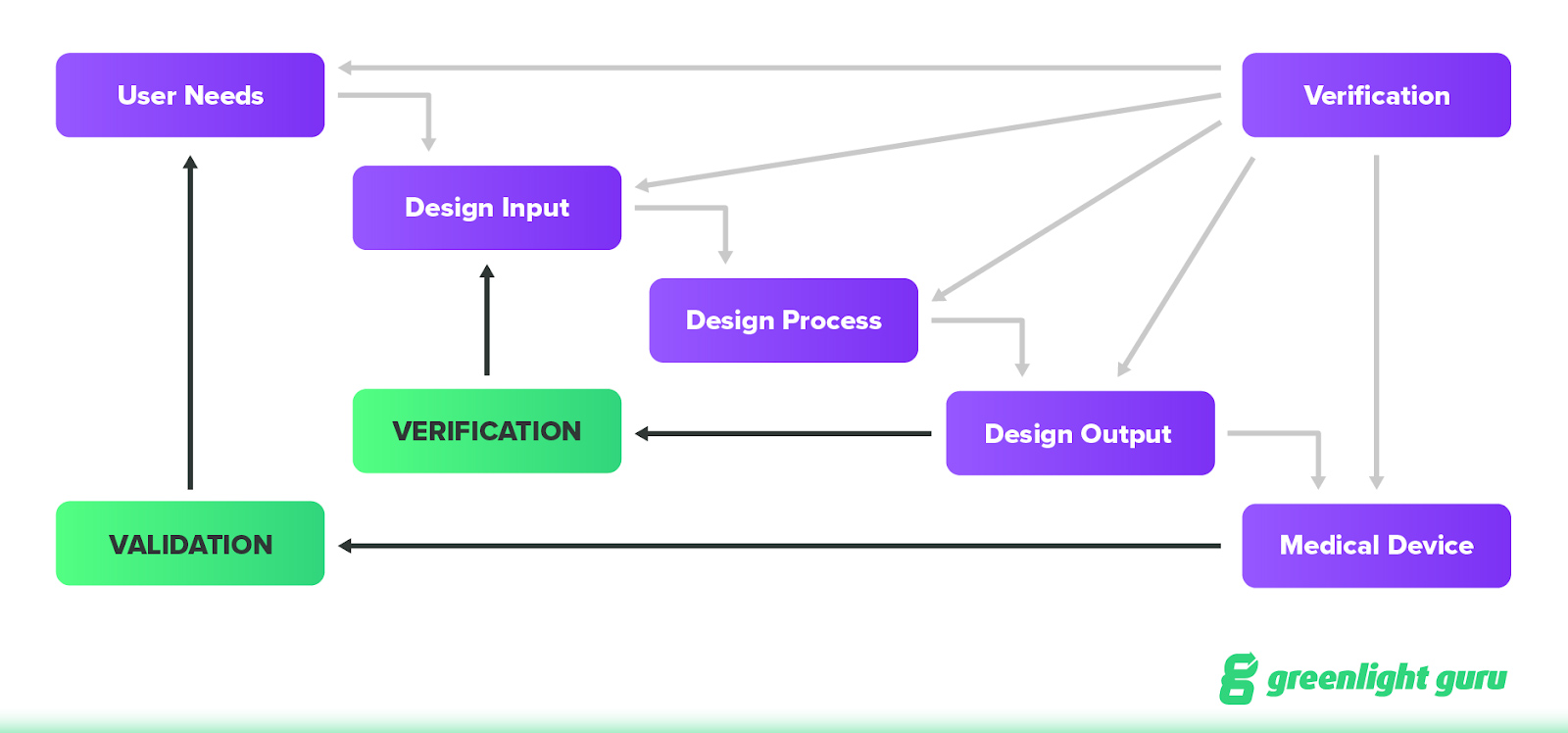
At a high level, the idea behind design controls is that they will not only improve the design of a device as the team goes through design and development, but also help to prevent future issues with a device once it’s placed on the market.
…

Add new comment