Tue, 08/07/2018 - 12:03
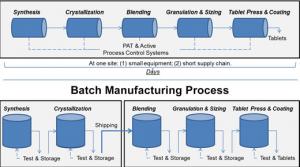
There’s new technology that can improve drug quality, address shortages of medicines, lower drug costs, and bring pharmaceutical manufacturing back to the United States. At the U.S. Food and Drug Administration (FDA), we’re focused on propelling…
Tue, 02/06/2018 - 12:01
In recent days, the U.S. Food and Drug Administration (FDA) has committed to several new policies that will modernize the agency’s approach to regulation in the medical device system.
For instance, we announced our intention to propose an…
Tue, 01/16/2018 - 12:01
Twice a year the federal government publishes the Unified Agenda of Federal Regulatory and Deregulatory Actions (Unified Agenda), which provides the American public with insight into regulations under development or review throughout the federal…Tue, 10/24/2017 - 12:01
The FDA has a long history of supporting patient access to investigational new treatments. This includes working with drug and device companies through the clinical trial process that may lead to FDA approval of the treatment. We also offer…
Wed, 07/19/2017 - 12:03
It is incumbent upon the U.S. Food and Drug Administration (FDA) to ensure that we have the right policies in place to promote and encourage safe and effective innovation that can benefit consumers, and adopt regulatory approaches to enable the…