All Features

Andreas Engelhardt
An international standard that specifies requirements for an occupational health and safety (OH&S) management system, ISO 45001:2018—“Occupational health and safety management systems–requirements” replaces OHSAS 18001 as the primary OH&S standard used internationally. It follows other…

Grant Ramaley
The Dental Trade Alliance learned from its members in February 2018 that the Canadian Health Ministry (“Health Canada”) had contacted the Standards Council of Canada (SCC) and the British Standards Institution (BSI). Health Canada had ordered these certification bodies to stop issuing ISO 13485…

Richard Pazdur
During the past decade, advances in understanding of cancer biology have led to the development of targeted treatments that are more effective than the chemotherapies of the past century. These therapies are demonstrating response rates large in magnitude or response durations prolonged in early…

Mike Richman
‘Culture” is one of those business-speak words that’s used a lot, but for a good reason—having the right one is the key to unlocking your company’s quality potential. On the other hand, nothing will overcome a poor culture. Do you know which you have? We explored these issues during the Aug. 10,…
Scott Gottlieb
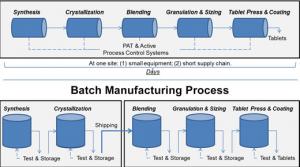
There’s new technology that can improve drug quality, address shortages of medicines, lower drug costs, and bring pharmaceutical manufacturing back to the United States. At the U.S. Food and Drug Administration (FDA), we’re focused on propelling these innovations, collectively referred to as…

Vanessa Burrows, Suzanne Junod, John Swann
During the early 20th century, Americans were inundated with ineffective and dangerous drugs, as well as adulterated and deceptively packaged foods.
A cosmetic eyelash and eyebrow dye called Lash Lure, for example, which promised women that it would help them “radiate personality,” in fact…

Matthew M. Lowe
The medical marijuana industry is being heralded as the new frontier in the life sciences, thanks to the potential of cannabis-derived products in treating ailments that range from chemotherapy-induced nausea to epilepsy and neuropathic pain. If you’re a startup in the industry, what does this mean…

Janet Woodcock
The staff of the U.S. Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) always tries to utilize cutting-edge science and up-to-date process management, befitting our stature as the global “gold standard” in drug regulation. Maintaining that standard requires us to…

Jon Speer
“I wish there was a way for the FDA to give me a heads-up about my stuff, prior to submission….”
That sentiment was really the basis behind the U.S. Food and Drug Administration’s (FDA) presubmission tool, as I was discussing recently with medical-device quality assurance and regulatory affiars…

Malvina Eydelman
The U.S. Food and Drug Administration’s (FDA) Breakthrough Devices Program is beginning to show important results for patients since it was established in late 2016 under the 21st Century Cures Act to help patients gain timely access to breakthrough technologies.
Consider Second Sight Medical…

AssurX
Recent FDA warning letters indicate that many drug manufacturers do not have their manufacturing in a state of current good manufacturing practices (CGMPs) control. During the first half of 2017, the FDA cited adulterated products and insanitary conditions as the two most common violations in drug…

Scott Gottlieb, Jeffrey Shuren
In recent days, the U.S. Food and Drug Administration (FDA) has committed to several new policies that will modernize the agency’s approach to regulation in the medical device system.
For instance, we announced our intention to propose an alternate approach to the traditional 510(k) clearance…

Jon Speer
Complaint handling continues to be one of the biggest reasons medical device companies receive 438s and warning letters from the U.S. Food and Drug Administration (FDA). Companies have a lot going on once a medical device has reached the market, and it can be challenging to keep up with…

Scott Gottlieb
Twice a year the federal government publishes the Unified Agenda of Federal Regulatory and Deregulatory Actions (Unified Agenda), which provides the American public with insight into regulations under development or review throughout the federal government. For the U.S. Food and Drug…

Lou Valdez, Dara Corrigan, Peter Stein
Regulatory experts from around the world, including the Food and Drug Administration (FDA), gathered recently to discuss issues such as regenerative medical products, international collaboration to fight antimicrobial resistance (AMR), and developing strategies to combat substandard or falsified…
Jon Speer
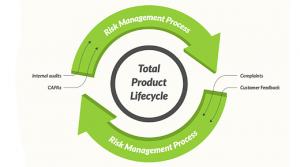
What exactly is a risk-based quality management system (QMS)? This is a timely topic to get into. In 2016, ISO 13485—“Medical devices”—“Quality management systems” was updated, and one of the key concepts presented is the idea of a risk-based QMS.
Historically, regulations have almost exclusively…

Grant Ramaley
I have written previously about the Medical Device Single Audit Program (MDSAP) created by the International Medical Device Regulators Forum (IMDRF). MDSAP is viewed as a single audit covering the United States, Canada, Brazil, Australia, and Japan. The intent was to establish one medical-device…

Mike Richman
QDL co-host Dirk Dusharme was on vacation for our Nov. 10, 2017, episode, but we ably covered for his absence with some thought-provoking stories and great guests. Let’s take a look:
“What Really Causes Workplace Stress” A multidisciplinary team of researchers at the University of Southern…

Brandon McFadden
The food labeling craze coupled with banner headlines about the dangers of gluten, genetically modified organisms (GMOs), and hormones are leading to increasingly absurd results.
For example, you can now buy “premium” water that’s not only free of GMOs and gluten but certified kosher and organic…

The QA Pharm
If I could summarize in one page the most important lessons I have learned in pharmaceutical quality assurance over the last 40 years, this is it.
When it comes to putting a procedure into written words, it doesn’t mean the words will be effective in getting people to follow the procedure.
The…

Dirk Dusharme @ Quality Digest
Our Oct. 27, 2017, episode of QDL looked at Ford, autonomous cars, and changes to FDA compassionate use rules.
“Ford Plans $14B in Cost Cuts as Part of New CEO’s Strategy”
Ford Motor Co’'s new CEO plans to cut $14 billion in costs, drop some car models, and focus the company’s resources on…
Scott Gottlieb
The FDA has a long history of supporting patient access to investigational new treatments. This includes working with drug and device companies through the clinical trial process that may lead to FDA approval of the treatment. We also offer expanded access programs that provide investigational…

Mike Richman
We cover a wide range of topics on QDL most weeks, but our latest episode, from Friday, Oct. 20, 2017, provided a steady drumbeat of technological detail. Here’s what we chatted about:
“Energy Harvested from Evaporation Could Power Much of U.S., Says Study” Renewal sources of energy like solar…

Jon Speer
How confident are you when it comes to design validation? Does this always involve clinical evaluation, or not? We’ve found that, like many other terms in medical device development, the two can end up getting confused. When do you use one or the other?
There tends to be a lack of clarity out…

Ann Cleland
|nids=22799|quantity=1-->
If your hospital or clinic uses a Windows 7-based version of a Siemens PET/CT or SPECT system, it could be vulnerable to attack by a relatively low-skill hacker, according to a July 26, 2017, security advisory from the company.
The Industrial Control System Cyber…