Big Data: Your Health vs. Your Privacy

For centuries, medical procedures, prescriptions, and other medical interventions have been based largely on experience—what is known about a set of symptoms.

For centuries, medical procedures, prescriptions, and other medical interventions have been based largely on experience—what is known about a set of symptoms.

Great quality is pretty much the same everywhere, but the cost of poor quality is not equivalent from industry to industry. For example, it’s conceivable (but I hope not probable) that this article may turn out to be a real bomb, or worse, a complete snoozer.

Within the life science industry, federal and industry regulations have prompted the need for compliance, and that trend has only increased in magnitude and complexity.

As the United States struggles with rising healthcare costs, reducing the amount of money pharmaceutical companies spend dealing with regulation, while at the same time meeting drug safety requirements, would seem to be competing interests.

Compliance to U.S. Food and Drug Administration (FDA) regulations has come a long way in the past 30 years. Here are the main changes. Have they affected your business?

Many industries have no clear boundary between safety and quality culture. In fact, they are often closely integrated.
The global wellness industry is doing superbly, thank you very much. In recent years, it grew a healthy 12.8 percent, becoming a $4.2 trillion market.
In this episode we look at lessons learned (or not) from GE, the difference between ISO and FDA “requirements,” and this year's Baldridge recipients.
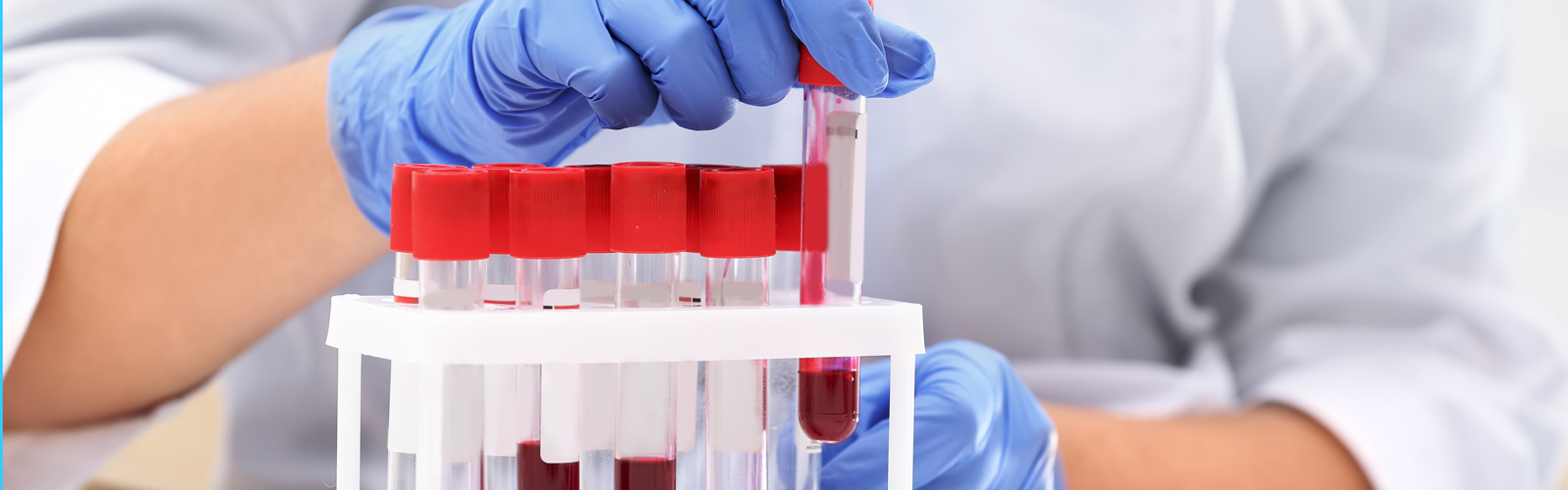
BioBridge Global (BBG) is a parent organization for four subsidiary organizations, three of which are involved in production activities, and they’re all around regenerative medicine, including blood components, clinical laboratory testing, and cell and tissue therapi
© 2025 Quality Digest. Copyright on content held by Quality Digest or by individual authors. Contact Quality Digest for reprint information.
“Quality Digest" is a trademark owned by Quality Circle Institute Inc.