
Tue, 12/19/2017 - 12:03
Corrective and preventive action (CAPA) is an important process for your medical device company. In fact, the U.S. Food and Drug Administration (FDA) states in its Quality System Inspection Technique (QSIT) guide, “One of the most important quality…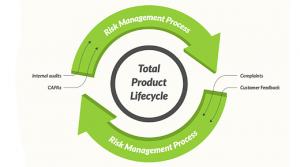
Mon, 11/27/2017 - 12:02
What exactly is a risk-based quality management system (QMS)? This is a timely topic to get into. In 2016, ISO 13485—“Medical devices”—“Quality management systems” was updated, and one of the key concepts presented is the idea of a risk-based QMS…
Tue, 10/17/2017 - 12:03
How confident are you when it comes to design validation? Does this always involve clinical evaluation, or not? We’ve found that, like many other terms in medical device development, the two can end up getting confused. When do you use one or the…
Tue, 08/29/2017 - 12:03
Medical device startups tend to share many common issues. They are usually striving for better resources (such as people and capital) as well as the knowledge and expertise required to deal with the U.S. Food and Drug Administration (FDA) and…
Tue, 03/28/2017 - 12:02
If you’re in the medical device industry, you may think that design controls are a confusing imposition on your processes. But they’re a necessary part of your requirements as a medical device developer, and I’ve noticed that this area tends to be… Key Challenges for Risk Management in Medical Device DevelopmentA proactive approach in a high-risk sector
Mon, 02/13/2017 - 12:01
If you’re in the business of developing medical devices, then risk and risk management become terms synonymous with your daily operations. Your overall task is to bring a device to market that not only provides a needed function to a patient, but… Six Tips to Make Sure Your 510(k) Submission Is AcceptedPlanning and organization will help you avoid the FDA’s refusal
Tue, 08/23/2016 - 16:18
Did you know that during the first six months of 2015, 69 percent of 510(k) submissions were rejected the first time? And that up to 75 percent of first-time 510(k) submissions are regularly sent back? I heard this and thought it was a crazy… Eight Reasons Why Your Design Controls and Risk Management Processes FailEmbrace the total product life cycle
Tue, 07/05/2016 - 17:01
Design controls and risk management processes should be tools to ensure that medical devices are designed, developed, and manufactured to be safe and effective, and to address indications for use, too.
All too often, however, design controls and… Why FMEA Is Not ISO 14971Medical device risks aren’t solely a function of failure
Wed, 04/20/2016 - 16:46
If you’re still using failure mode and effects analysis (FMEA) as your methodology to capture medical-device risk management activities, then your risk management process is out of date. Let me tell you why.
Here’s the definition of “risk… How to Prepare for an FDA InspectionFive tips to help you get ready
Mon, 02/08/2016 - 14:51
During the past several years, the FDA has been more aggressive and active in performing medical device company inspections. This has led to a far greater number of companies receiving Form 483 warning letters and citations. FDA Form 483s are a…