All Features

Etienne Nichols
If you’re a medtech professional who’s been working with the quality system regulation (QSR) in the United States, then you’re probably familiar with the three terms the U.S. Food and Drug Administration uses for record-keeping requirements: 1) device master record (DMR), a compilation of records…

Bruce Hamilton
As years roll on, I’m noticing more parts of me breaking down: Teeth, eyes, knees, cardiovascular, stomach—the list keeps getting longer, as does the list of docs I see. I’m blessed to be living in an area with the world’s finest medical care and lucky that healthcare innovation (and Medicare) have…

Veronica Muzquiz Edwards
Health connects each one of us to one another. No matter where we are in the world, who we are, or what we do, the state of our health is a key determinant in our quality of life. Simply put, it’s our most valuable asset.
Individual health crises can be disastrously grim, and if not addressed…

Gabriel Popkin
They’re called per- and polyfluoroalkyl substances, or PFAS, a group of thousands of compounds that contain a chemical bond between fluorine and carbon. That bond has proved to be one of the most stable and unbreakable known to chemistry—a fact baked into the common nickname “forever chemicals,”…
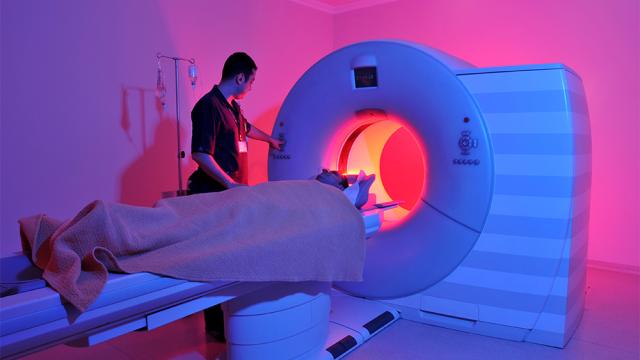
Rob Moorey
Equipment failures in healthcare can have serious consequences, including delays in diagnosis or treatment, scheduling disruptions, and patient safety risks. Health systems should empower clinical engineering teams with technology that helps identify potential failures. This will allow health…

Jessica Rector
Burnout is affecting every industry, company, and role. There are no exceptions.
Leaders often find themselves in the trenches, navigating through the chaos and driving their teams toward success. However, amidst the pursuit of goals and objectives, burnout remains a lurking enemy that can…
Etienne Nichols
In the United States, the Food and Drug Administration (FDA) is the federal agency tasked with regulating the medical device market and ensuring the safety and effectiveness of all devices for patients.
The FDA classifies medical devices by risk into three categories: Class I, Class II, and Class…

Stephanie Ojeda
An analysis of U.S. Food and Drug Administration (FDA) warning letters by the Food and Drug Law Institute reveals a perhaps not-so-surprising link between training gaps and FDA violations.
It’s one of several factors motivating companies to switch to automated training management software. The…

Aman Pandey
In the dynamic landscape of the life sciences industry, ensuring compliance with good manufacturing practices (GMP) is imperative to guarantee the safety, efficacy, and quality of pharmaceutical products.
One critical aspect of GMP is the analytical product quality review (APQR), an essential…

Morehouse Instrument Co.
In the healthcare sector, precision isn’t just a requirement. It’s a necessity where the margins for error are perilously thin, and the consequences of inaccuracy can be grave. At the heart of this precision lies the unassuming yet critical load cell, a device whose reliability is foundational to…

Dawn Bailey
The Center for Organ Recovery & Education (CORE), a 2019 Baldrige Award recipient, is a nonprofit organ procurement organization (OPO) in Pittsburgh with a federally designated service area encompassing a population of 5.5 million in western Pennsylvania, West Virginia, and one county in New…

Patrick Gale
Medical equipment is a necessary yet substantial investment for any health system. Making strategic decisions about these assets can be daunting in the face of shifting patient demand, financial uncertainty, and fast-changing cybersecurity risks.
Because clinical assets account for an average of…

Mike Figliuolo
If you have kids, you know the nauseating feeling of one of them going down for the count and having to rush them to the emergency room. I had that grim experience recently. What I learned from that ER visit is businesses can make very strong statements about how little they care about their…

Jones Loflin
When we talk about a lack of work-life balance, stress, or burnout, one of the things we’re actually saying to ourselves is that we feel we have no control over the outcome or our future. It can feel like the line between work and life has blurred into one big, overwhelming blob.
It’s time for a…
Alonso Diaz, Maria DiBari
The U.S. Food and Drug Administration (FDA) emphasizes the importance of being prepared for device recalls.
FDA product recalls are on the rise in the post-pandemic era. There has been a clear upward trend from 2021 through 2023, and medical devices ranked the highest of all product types. (See…

Maggie Overfelt
Michele Gelfand finds inspiration for new projects all around her: taking in the banter in a boardroom, speaking with taxi drivers when traveling, observing the interactions between physicians and nurses during an unexpected trip to the doctor. The idea for one of her most recent papers was sparked…

Jamie Bihary
An internal audit can be an overwhelming prospect, especially if you’re new to a company or internal auditing in general.
The MedTech space is huge, and even the standards that are meant to help, like ISO 13485:2016, cover a lot of ground.
So, if you’re part of the audit team in your company, and…

Stephanie Ojeda
Formal complaint management is a requirement in regulated industries such as medical device and pharmaceuticals under U.S. Food and Drug Administration (FDA) and other international regulations.
The FDA mandates that medical device companies, for example, designate a formal unit for managing…

Michael King
In the ever-evolving life-sciences industry, market share is fiercely contested and companies must continuously optimize their operations to maintain their competitive edge.
Modern technologies and intelligence-driven solutions are revolutionizing how organizations work, empowering them to elevate…

Meg Sinclair
At Qualio, our mission is to help life science companies embed robust digitized quality to get their critical products to market at rapid speed and keep them there. And because the Qualio+ team combines over a century of collective quality and regulatory experience from within the life science…

Kristi McDermott
Technology has reshaped the healthcare industry, empowering clinicians, technicians, and executives to better serve patients and achieve their goals. However, technology doesn’t eliminate the need for human oversight and management. Healthcare technology will continue to advance at a rapid pace in…

Alonso Diaz, Maria DiBari
Inspections by the U.S. Food and Drug Administration (FDA) are on the rise after the nation has recovered from the Covid-19 pandemic. Domestic inspections showed a drop in 2020 due to state health guidelines around quarantine.
The rise has more than doubled within three years of post-pandemic…

Scott A. Hindle, Douglas C. Fair
So far in this series our focus has remained on statistical process control (SPC) in manufacturing. We’ve alternated between more traditional uses of SPC that remain relevant in this digital era and discussing uses of SPC and its related techniques that are enabled by the marvels of modern…

Kari Miller
Traditionally, quality management in the pharmaceutical industry has strayed away from artificial intelligence (AI) for fear of setting it loose with such sensitive information. They have been cautious of implementing an additional element of intelligence into their process. But will organizations…
Brad Jobe
Artificial intelligence (AI) has the potential to reshape the healthcare industry. There is a massive amount of healthcare data available for AI to process. Nearly one-third of the world’s data volume is generated by the healthcare industry, and the volume of big data is projected to increase…