All Features
Jón Bergsteinsson
Clinical investigations play an important role in your journey of bringing a medical device to market. While the relevant standards are often perceived as difficult and complex, having a good grasp of them makes the process less confusing.
Understanding ISO 14155:2020 is essential. It’s a guide to…
Rob Moorey
Growing medical equipment inventories and increasing technical complexity are demanding more than ever from the clinical engineering teams responsible for maintaining clinical assets. Simultaneously, a shrinking talent pool of biomedical equipment technicians (BMETs) could lead to large staffing…

Stephanie Ojeda
In December 2023, the U.S. Food and Drug Administration (FDA) expects to issue its long-awaited overhaul of its Quality System Regulation (QSR). The biggest change is that the new Quality Management System Regulation (QMSR) will harmonize with ISO 13485 for medical device quality management. With…
Steve Thompson
If you’ve ever enjoyed the experience of an audit or inspection, then you know it’s about as much fun as having your wisdom teeth extracted. As painful as audits and inspections may be, they are necessary to bring needed medical products to market and monitor them to protect consumers and patients…

ISO
Healthcare administrators find themselves at the fore of a demanding and transformative field, where the pursuit of excellence in patient care is nonnegotiable. In a health industry landscape facing evolving regulations, escalating costs, and an increasing emphasis on patient outcomes, the need for…

Stephanie Hinton
If you’re conducting a clinical investigation of a medical device in a European Union member state, you will be required to submit a clinical investigation report (CIR) along with a summary of the CIR to that member state.
The European Union Medical Device Regulation (EU MDR) lists this as one of…

Kelley Jacobsen
In the wake of the Covid-19 pandemic, medical device supply chains are one of the top priorities for health system leaders. Amid supply chain disruptions during the pandemic, hospitals scrambled to find enough devices to keep up with unprecedented demand. The global crisis revealed gaps in standard…

Etienne Nichols
Supply chain management is crucial to any medtech company’s ability to deliver safe, effective, and high-quality devices to their customers.
But as anyone in the industry can tell you, consistently getting the products and services you need to manufacture your devices is harder than it sounds. In…
Chris Bush
Untitled Document
The U.N. recognizes privacy as a fundamental human right, and nowhere is this more important than in medical data. That’s why both the U.S. and the EU have regulations in place that govern the collection, storage, and use of patient data in healthcare.
In the U.S., there is the…
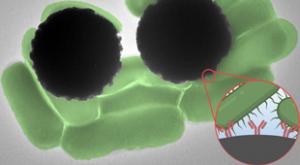
Jennifer Chu
Getting blood test results can take anywhere from a day to a week, depending on what a test is targeting. The same goes for tests of water pollution and food contamination. And in most cases, the wait time has to do with time-consuming steps in sample processing and analysis.
Now, MIT engineers…

NIST
Magnetic resonance imaging (MRI) machines can clearly view non-bony parts of the body—soft tissue such as the brain, muscles, and ligaments—as well as detect tumors, making it possible to diagnose many diseases and other conditions. However, the powerful magnets in conventional MRI machines make…

Lindsey Walker
In the quickly changing industrial landscape, firms continue to place a high premium on safety. Innovative approaches to improving industrial safety have been made possible by technological advancements. One particularly revolutionary option is computerized maintenance management system (CMMS)…
Etienne Nichols
On Feb. 23, 2022, the U.S. Food and Drug Administration (FDA) released its proposed rule for the new Quality Management System Regulation (QMSR). The proposed QMSR will be the result of aligning the current good manufacturing practice (cGMP) requirements of the FDA’s Quality System Regulation (QSR…

Etienne Nichols
Amedical device company is expected to deliver innovative, life-changing devices while ensuring compliance and achieving true quality. This task bears loads of responsibility—all of which must be kept and documented within your quality management system (QMS).
A QMS contains everything that…

Aaron Smith
A successful company can’t run without happy and motivated employees. One way you can achieve that is by improving your employees’ uptime. Uptime refers to your employees’ freedom to pursue personal and occupational growth without the burden of preventable injuries. Here is everything you need to…

Stephanie Ojeda
Design controls are a frequent citation in 483 observations and warning letters from the U.S. Food and Drug Administration (FDA). In fact, the agency has noted a large proportion of past recalls that could have been prevented with design controls.
FDA guidance also makes an explicit link between…

Gleb Tsipursky
The unemployment rate is surprisingly low, at 3.7%, shocking economists who expected a slowdown in hiring and rising unemployment rate. Frontline work, such as healthcare, led job growth. Frontline workers are in high demand, and the competition for their services is fierce. Yet wage growth cooled…

Etienne Nichols
The goal of your MedTech company’s supplier management process should be to ensure a consistent supply of high-quality parts and components that conform to your specifications.
But achieving that goal is easier said than done, and it depends heavily on whether you take a risk-based approach to…

Stephanie Ojeda
Effective complaint handling is fundamental to life-sciences quality management, with implications for operations, product design, risk management, and more.
It’s also critical to ISO 9001, FDA Quality System Regulation (QSR), and EU Medical Device Regulation (MDR) compliance.
Manufacturers that…
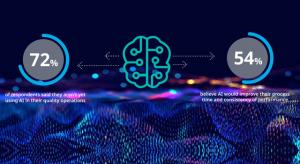
Michael King
Medical companies work in an environment of ever-increasing challenge and complexity. Global regulations continue to evolve with advancements in technology and variations in requirements from country to country. This brings unavoidable technical complexity to daily tasks of quality and regulatory…

Mike Morini
Following the turmoil of the pandemic, many salaried employees have had the opportunity to work remotely and enjoy newfound flexibility in their day-to-day work lives. For many, the ability to be more in control over where and when they work has been life-changing, enabling them to find better work…

Silke von Gemmingen
Rigid endoscopes are used in medical diagnostics and therapy to examine body cavities and hollow organs, and to perform minimally invasive procedures. To avoid risks for patients and medical staff, it’s essential that the devices function perfectly. Reliable quality testing is intended above all to…

Melissa Phillips
The levels of contaminants in our food supply are, generally, decreasing. That’s the good news.
But we still need to measure those contaminants and make sure our food is safe. And measuring tiny things (and big things) is what we do best here at NIST.
In our food safety program, we’re studying all…
Etienne Nichols
Medical device companies must have established risk management processes that comply with ISO 14971. It doesn’t matter whether you’re developing medical devices in the U.S., EU, Canada, or elsewhere. Every international regulatory agency you’ve ever heard of accepts ISO 14971.
ISO 14971 is a good…

Ophir Ronen
From the health histories of patients to the effectiveness of different healthcare services, hospitals are sitting on a treasure trove of historical data. Unfortunately, most of these data go unused, often because they are so difficult to store or format for actionable use.
Due to inconsistencies…