All Features

Andreas Janz, Horacio Falcão
Failing to identify faults seems to be a prevalent issue nowadays, considering recent reports of quality control problems at Boeing and numerous recalls by car manufacturers, to name a few. Yet, excessive fault-finding occurs when the ability to pinpoint faults—a beneficial behavior—is taken too…

Creaform
When it comes to aircraft, poorly documented dents can lead to more significant problems, potentially compromising structural integrity or performance. Dents can trap moisture and lead to corrosion. The stress they generate can initiate fatigue cracks. Their effects on the structure can also affect…

James Chan
Thoughtful planning is the cornerstone of an effective preventive maintenance program. Your maintenance plan is the framework for everything from building a maintenance schedule and automating inventory management to streamlining work order management and improving interdepartmental communication…

Jones Loflin
I love gardening and growing fresh vegetables. Recently I had checked in on my Brussels sprouts seedlings and, well, they looked awful! The reason quickly became obvious to me: I had overwatered them.
I was so excited about growing Brussels sprouts this year, I didn’t want anything to happen to…

Matt Fieldman
If you look around your plant floor, I’m sure the layout, equipment, and technologies are different today from what they were 10—or even five—years ago. You’ve put time, effort, and money into improving every aspect of your operations. You’ve cut costs and made investments where they were necessary…

Etienne Nichols
If you’re a medtech professional who’s been working with the quality system regulation (QSR) in the United States, then you’re probably familiar with the three terms the U.S. Food and Drug Administration uses for record-keeping requirements: 1) device master record (DMR), a compilation of records…

David Isaacson
For any manufacturer, ensuring well-run, successful processes and protocols is the backbone of a successful and profitable operation. Yet, despite the most well-laid plans, circumstances can change and manufacturers may sidestep best practices in the race to meet demand. Still, ensuring quality,…

William A. Levinson
The International Accreditation Forum (IAF) and ISO have published a joint communiqué to require organizations to “consider” climate change in the context of risks and opportunities relevant to the management system.
Although this is pursuant to the London Declaration, which has goals for…

Stephanie Ojeda
Look through even a few FDA warning letters and you’re likely to find violations related to change management.
For instance, a recent warning letter from the U.S. Food and Drug Administration cited a pharmaceutical manager for changing drug components without justification. Another noted a lack of…

Stefano Puntoni, Bart De Langhe
Most businesses rightly see data as a source for making better decisions. But the conventional data-driven approach often falls short because of common errors made by the decision-makers. Many leaders rely excessively on existing data that might not always address the issue at hand, or they pass…

Veronica Muzquiz Edwards
Health connects each one of us to one another. No matter where we are in the world, who we are, or what we do, the state of our health is a key determinant in our quality of life. Simply put, it’s our most valuable asset.
Individual health crises can be disastrously grim, and if not addressed…

NIST
To combat global warming, companies are building direct air capture (DAC) facilities worldwide to remove carbon from the atmosphere. The National Institute of Standards and Technology (NIST) has developed a new method for testing the materials used in these plants to capture carbon. The agency…

James Chan
The plan-do-check-act (PDCA) cycle plays a central role in fostering improvement by facilitating a structured and ongoing approach to problem-solving. Because the PDCA cycle is ongoing, it also plays a central role in helping organizations navigate shifts in the economic climate, align with new…

Gleb Tsipursky
In the evolving landscape of workplace dynamics, the trend toward revoking employee flexibility and mandating a return to the office (RTO) is gaining traction among corporations, as evidenced by significant players like Boeing enforcing near-full-week office attendance. Leaders often cite the…

Andy J. Yap, Winnie Jiang, Mark Mortensen, Spencer Harrison
Imagine a world where your boss could be fined for contacting you after hours. California is considering a law to make this a reality.
The “right to disconnect” movement is gaining traction globally, with Australia joining France, Italy, Slovakia, Luxembourg, Portugal, and the Canadian province…

Daniel Marzullo
When was the last time you asked your clients for genuine feedback on working together, beyond just revisions on projects and deliverables?
Over the years, I’ve found it incredibly beneficial to do quarterly check-ins with every client to get a sense of how things are going.
Not only does this…

Knowledge at Wharton
Nano Tools for Leaders, a collaboration between Wharton Executive Education and Wharton’s Center for Leadership and Change Management, are fast, effective tools that you can learn and start using in less than 15 minutes, with the potential to significantly affect your success and the engagement and…

Gabriel Popkin
They’re called per- and polyfluoroalkyl substances, or PFAS, a group of thousands of compounds that contain a chemical bond between fluorine and carbon. That bond has proved to be one of the most stable and unbreakable known to chemistry—a fact baked into the common nickname “forever chemicals,”…

James Chan
Preventive maintenance (PM) is an approach to asset and facilities management that relies heavily on planning, coordination, and collaboration. These features are essential to performing preventive maintenance activities such as routine inspections, preemptive equipment maintenance, and parts…

Luk Van Wassenhove, Akhil Bhardwaj, Henk Akkermans
Disasters can act as brutal audits of organizations to which even venerable institutions like Boeing are subject. In the wake of two fatal crashes, as well as a midflight door blowout, Boeing has shaken up its C-suite and pledged to prioritize safety over profit.
Will these measures solve its…

James Chan
Preventive maintenance (PM) is a proactive maintenance strategy built on calendar-based maintenance tasks, regular inspection, and preemptive repair of physical assets. Physical assets may refer to equipment, production machinery, and operational facilities. Preventive maintenance tasks are…

Katie Gilbert
Researchers at Stanford Graduate School of Business have pushed past the limitations of A/B testing into another area of experimentation focused on “multi-armed bandits.” Mohsen Bayati, a professor of operations, information, and technology who has been exploring these problems for the past 15…

Pierre-Nicolas Disser, Megan Wallin-Kerth
QIMA, previously called AsiaInspection, is known for not only making inspection and certifications easier, but also increasing accessibility via a convenient digital platform and strong focus on compliance. Both SMEs and e-commerce businesses have benefited from this increase in affordability and…
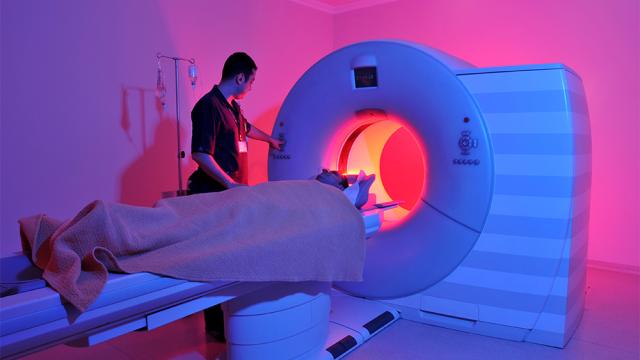
Rob Moorey
Equipment failures in healthcare can have serious consequences, including delays in diagnosis or treatment, scheduling disruptions, and patient safety risks. Health systems should empower clinical engineering teams with technology that helps identify potential failures. This will allow health…

ISO
From small family-run companies to tech giants, the business world is changing at an unrelenting pace. Amid a constantly evolving economic landscape and sometimes dizzying technological advances, one thing remains constant: the need to maintain the highest level of quality that endures over time.…