All Features

John O'Kelly
Warehouses are the backbone of supply chains, ensuring that goods move efficiently from suppliers to consumers. However, the physical demands of warehouse work—heavy lifting, repetitive motions, and prolonged standing—can take a toll on employees, leading to fatigue, injuries, and long-term strain…

ISO
How do health and safety incidents affect your business? If a worker is injured or becomes ill, what kind of disruption does it cause? Is your productivity affected? What’s the effect on other workers in terms of workload or psychological health and well-being?
People are the foundation of every…

IDS Imaging Development Systems
The slit-lamp examination is one of the most important diagnostic techniques in ophthalmology. It enables a detailed examination of the anterior, middle, and posterior segments of the eye. Ophthalmologists can use it to recognize the smallest changes, anomalies, or damage. This procedure is used…
Matt McFarlane
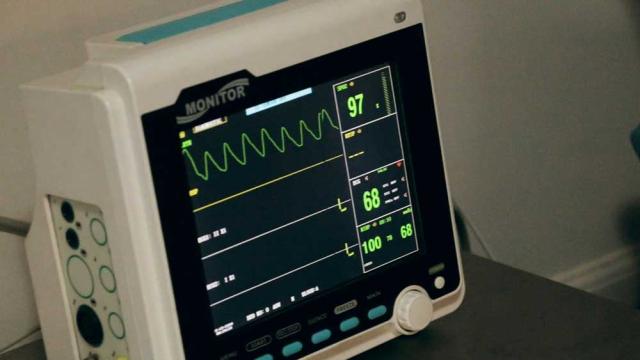
The medical device industry is growing. Data from KPMG predict that global annual sales will rise by 5% per year to reach just under $800 billion by 2030. New technology, new opportunities, and, as always, the promise of improving patient outcomes around the world are major drivers of growth within…
Etienne Nichols
Have you ever wondered what your medtech company looks like from the point of view of a U.S. Food and Drug Administration investigator? Well, this is your chance to find out.
Greenlight Guru invited Vincent Cafiso, a former FDA investigator, to the Global Medical Device Podcast to share his…

Stephanie Ojeda
Quality risk management (QRM) has become a crucial tool for ensuring regulatory compliance worldwide. It plays a central role in ISO management system standards and regulations, as well as the EU Medical Device Regulation (MDR/IVDR), FDA 21 CFR 820, and ICH Q10 in the pharmaceutical and biotech…

Stephanie Ojeda
In April 2018, the U.S. Food and Drug Administration (FDA) approved the first artificial intelligence-powered diagnostic system, a software program used to detect diabetes-related vision loss.
Since then, the industry has seen explosive growth of AI in medical device manufacturing, which is…

Ilana J. Sprongl
Back in 2023, only 15% of businesses had adopted AI-augmented software testing tools. By 2027, that number is expected to leap to 80%. The reason behind this rapid adoption is clear. The complexity of modern software and products is skyrocketing, and with it, the risks associated with quality…

Etienne Nichols
Compliance with industry regulations and standards is a fundamental part of medtech. Without proper medical device compliance, companies risk patient harm, litigation, and reputational damage.
Fortunately, compliance with medical device regulations and standards is not an impossible task. A…
Victoria Alestra
In regulated industries like pharmaceuticals, medical devices, and food manufacturing, compliance is crucial for operational excellence. A validated quality management system (QMS) is key to maintaining this compliance. Let’s explore how QMS software streamlines validation and ensures regulatory…
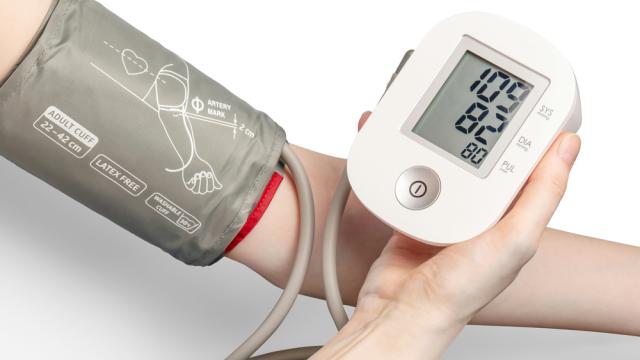
Chris Rush
While clinical trials are the gold standard for generating clinical data to use as evidence of your medical device’s safety and effectiveness, they are by no means the only way to gather clinical evidence.
Real-world data (RWD), which typically come from routine healthcare delivery or…

Stephanie Ojeda
In the highly regulated world of life sciences, data integrity isn’t optional; it’s essential. The ALCOA principles—attributable, legible, contemporaneous, original, and accurate—provide a foundational framework for ensuring data are reliable and trustworthy.
With the rise of digital…

Joshua Zable
Everyone has their own favorite graph type or visual tool. I’m not ready to declare this my favorite yet, but this oldie but goodie has got to get more time and attention. That’s right: I’m talking about control charts with stages, also sometimes called before/after control charts.
If you’re not…

Zach Winn
Most doctors go into medicine because they want to help patients. But today’s healthcare system requires that they spend hours each day on other work—searching through electronic health records (EHRs), documenting, coding and billing, gaining prior authorization, and evaluating services—that often…

Sachin Waiker
‘Clinician burnout is a critical issue to understand and address,” says Mohsen Bayati, a professor of operations, information, and technology at Stanford Graduate School of Business. The condition is thought to affect nearly half of all U.S. doctors, at a cost of about $4.6 billion annually due to…

ISO
Cybersecurity has become increasingly critical in the digital age as organizations across all sectors face growing threats from cybercriminals.
Imagine that hackers breached a small healthcare practice through “phishing”—sending a scam email and gaining access to sensitive patient data, including…

Saurabh Joshi Shripad
Before the ICH Harmonized Tripartite Guideline Q9—“Quality risk management”—was introduced in 2005, the pharmaceutical industry was evolving but lacked a structured, scientific, and systematic approach. Various stakeholders, including the industry, regulators, and patient rights groups, recognized…
Laura Velásquez Herrera
With its roots in compassion and humanity, the healthcare sector might seem an unlikely place for artificial intelligence (AI) to play a big role. Yet as we look deeper into the complex processes that build our medical systems, we uncover a multitude of ways that AI could revolutionize patient care…
James Chan
The U.S. Food and Drug Administration (FDA) is the country’s chief agency for regulating the manufacture, marketing, and distribution of critical consumer goods including food, cosmetics, medical devices, biological products, and pharmaceuticals. The FDA provides direct oversight of the businesses…
Jennifer King
Many people don’t realize just how long AI has been around in the healthcare industry—and are surprised to find out that it’s something that’s been relied on for 50 years already.
MYCIN, a computer-based model with machine learning capabilities, was developed by a team of researchers at Stanford…

Robyn Coward, Brian Brooks
This year, the Medical Device Innovation Consortium held an Excellence in Quality Summit where it was promised that participants would receive a “unique opportunity to learn adoptable cutting-edge practices to maximize the impact of investing in quality across [their] total product life cycle.”…

Etienne Nichols
On Jan. 31, 2024, the U.S. Food and Drug Administration (FDA) released its final rule for the new Quality Management System Regulation (QMSR).
The new QMSR is the result of aligning the current good manufacturing practice (cGMP) requirements of the FDA’s quality system regulation (QSR) with the…
Chris Rush
Ensuring the accuracy and security of clinical data, as well as compliance with good clinical practice (GCP), will in large part determine the success of your study and regulatory submission to the U.S. Food and Drug Administration (FDA). Data management and reporting are essential practices when…
Mike King
Historically, the sensitive nature of personal and company proprietary information held in life sciences quality management systems (QMS) has been a factor for quality management teams’ reluctance to adopt AI. Add to that the complex global regulatory environment and the penalties of noncompliance…

ISO
When a patient comes into a clinic or hospital, healthcare practitioners have all the tools at their disposal to conduct thorough examinations. However, when they see a patient online, they may lack the necessary equipment to conduct the visit properly. One reason for this is that virtual care…